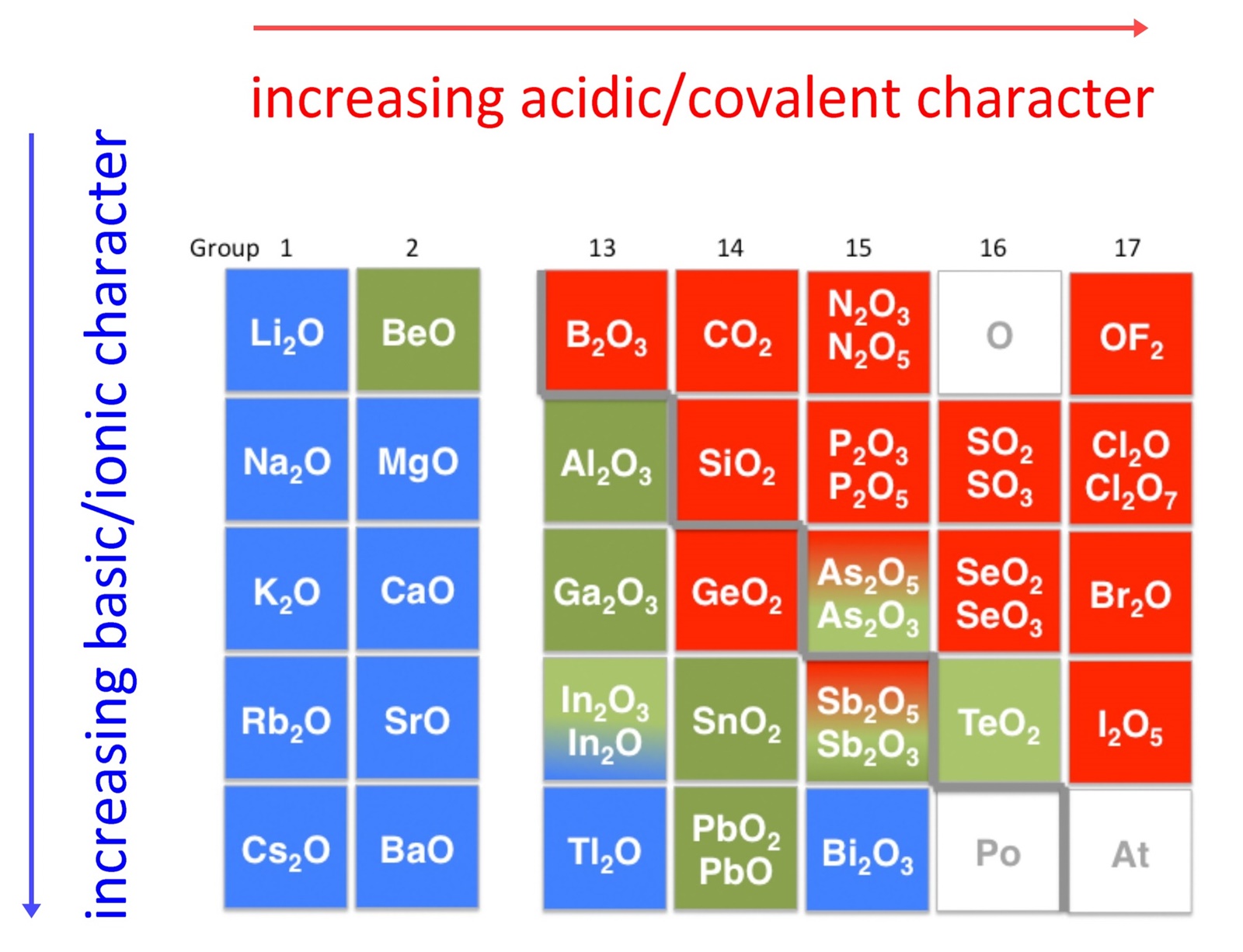
Iron Iii Oxide Acid Or Base . chemical properties of iron(iii) oxide reaction with acids. It is also referred to as ferric oxide, red iron oxide, iron trioxide, and oxo (oxoferriooxy) iron. Iron(iii) oxide reacts with acids, such as hydrochloric acid, to form iron(iii) chloride and water. In phosphorous acid, the two hydrogen atoms. iron(iii) oxide has the chemical formula {eq}fe_{2}o_{3} {/eq}. ferric oxide, also known as iron(iii) oxide, is an amphoteric oxide of iron. iron iii oxide is generally basic and it forms a weak base solution when it gets completely dissolved in water. iron (iii) oxide is a compound that appears in at least four different polymorphs: Some of the metals form very common ions which have latin names that are in common use,. phosphorus(iii) oxide is unlikely to be reacted directly with a base. so fe +2 is iron (ii) and fe +3 is iron (iii).
from dxosytfoi.blob.core.windows.net
iron(iii) oxide has the chemical formula {eq}fe_{2}o_{3} {/eq}. Iron(iii) oxide reacts with acids, such as hydrochloric acid, to form iron(iii) chloride and water. phosphorus(iii) oxide is unlikely to be reacted directly with a base. iron (iii) oxide is a compound that appears in at least four different polymorphs: chemical properties of iron(iii) oxide reaction with acids. ferric oxide, also known as iron(iii) oxide, is an amphoteric oxide of iron. iron iii oxide is generally basic and it forms a weak base solution when it gets completely dissolved in water. Some of the metals form very common ions which have latin names that are in common use,. It is also referred to as ferric oxide, red iron oxide, iron trioxide, and oxo (oxoferriooxy) iron. so fe +2 is iron (ii) and fe +3 is iron (iii).
Iron(Iii) Oxide Compound Name at Matthew Pina blog
Iron Iii Oxide Acid Or Base ferric oxide, also known as iron(iii) oxide, is an amphoteric oxide of iron. iron (iii) oxide is a compound that appears in at least four different polymorphs: Iron(iii) oxide reacts with acids, such as hydrochloric acid, to form iron(iii) chloride and water. so fe +2 is iron (ii) and fe +3 is iron (iii). ferric oxide, also known as iron(iii) oxide, is an amphoteric oxide of iron. chemical properties of iron(iii) oxide reaction with acids. It is also referred to as ferric oxide, red iron oxide, iron trioxide, and oxo (oxoferriooxy) iron. iron(iii) oxide has the chemical formula {eq}fe_{2}o_{3} {/eq}. phosphorus(iii) oxide is unlikely to be reacted directly with a base. In phosphorous acid, the two hydrogen atoms. iron iii oxide is generally basic and it forms a weak base solution when it gets completely dissolved in water. Some of the metals form very common ions which have latin names that are in common use,.
From www.inoxia.co.uk
Buy Iron (III) Oxide at Inoxia Ltd Iron Iii Oxide Acid Or Base so fe +2 is iron (ii) and fe +3 is iron (iii). Iron(iii) oxide reacts with acids, such as hydrochloric acid, to form iron(iii) chloride and water. iron iii oxide is generally basic and it forms a weak base solution when it gets completely dissolved in water. chemical properties of iron(iii) oxide reaction with acids. It is. Iron Iii Oxide Acid Or Base.
From www.slideserve.com
PPT Combustion analysis PowerPoint Presentation, free download ID9468256 Iron Iii Oxide Acid Or Base phosphorus(iii) oxide is unlikely to be reacted directly with a base. Some of the metals form very common ions which have latin names that are in common use,. so fe +2 is iron (ii) and fe +3 is iron (iii). In phosphorous acid, the two hydrogen atoms. ferric oxide, also known as iron(iii) oxide, is an amphoteric. Iron Iii Oxide Acid Or Base.
From cevzuiig.blob.core.windows.net
Iron 3 Oxide State Of Matter at Marcus Ellis blog Iron Iii Oxide Acid Or Base ferric oxide, also known as iron(iii) oxide, is an amphoteric oxide of iron. Some of the metals form very common ions which have latin names that are in common use,. It is also referred to as ferric oxide, red iron oxide, iron trioxide, and oxo (oxoferriooxy) iron. Iron(iii) oxide reacts with acids, such as hydrochloric acid, to form iron(iii). Iron Iii Oxide Acid Or Base.
From stock.adobe.com
iron III Oxide Properties and Chemical Compound Structure Stock Vector Adobe Stock Iron Iii Oxide Acid Or Base iron iii oxide is generally basic and it forms a weak base solution when it gets completely dissolved in water. chemical properties of iron(iii) oxide reaction with acids. iron (iii) oxide is a compound that appears in at least four different polymorphs: so fe +2 is iron (ii) and fe +3 is iron (iii). Some of. Iron Iii Oxide Acid Or Base.
From chemistry-europe.onlinelibrary.wiley.com
Reduced Amino Acid Schiff Base‐Iron(III) Complexes Catalyzing Oxidation of Cyclohexane with Iron Iii Oxide Acid Or Base In phosphorous acid, the two hydrogen atoms. Iron(iii) oxide reacts with acids, such as hydrochloric acid, to form iron(iii) chloride and water. iron (iii) oxide is a compound that appears in at least four different polymorphs: Some of the metals form very common ions which have latin names that are in common use,. phosphorus(iii) oxide is unlikely to. Iron Iii Oxide Acid Or Base.
From www.inoxia.co.uk
Buy Iron (III) Oxide at Inoxia Ltd Iron Iii Oxide Acid Or Base chemical properties of iron(iii) oxide reaction with acids. iron(iii) oxide has the chemical formula {eq}fe_{2}o_{3} {/eq}. phosphorus(iii) oxide is unlikely to be reacted directly with a base. iron iii oxide is generally basic and it forms a weak base solution when it gets completely dissolved in water. In phosphorous acid, the two hydrogen atoms. It is. Iron Iii Oxide Acid Or Base.
From www.sciencephoto.com
Iron(III) Oxide Stock Image C030/8153 Science Photo Library Iron Iii Oxide Acid Or Base phosphorus(iii) oxide is unlikely to be reacted directly with a base. iron (iii) oxide is a compound that appears in at least four different polymorphs: ferric oxide, also known as iron(iii) oxide, is an amphoteric oxide of iron. iron(iii) oxide has the chemical formula {eq}fe_{2}o_{3} {/eq}. Iron(iii) oxide reacts with acids, such as hydrochloric acid, to. Iron Iii Oxide Acid Or Base.
From cerojfxl.blob.core.windows.net
Iron Iii Oxide Examples at Elaine Ortiz blog Iron Iii Oxide Acid Or Base It is also referred to as ferric oxide, red iron oxide, iron trioxide, and oxo (oxoferriooxy) iron. iron iii oxide is generally basic and it forms a weak base solution when it gets completely dissolved in water. In phosphorous acid, the two hydrogen atoms. chemical properties of iron(iii) oxide reaction with acids. Some of the metals form very. Iron Iii Oxide Acid Or Base.
From www.pw.live
Iron III Oxide Formula, Structure, Properties, Uses Iron Iii Oxide Acid Or Base It is also referred to as ferric oxide, red iron oxide, iron trioxide, and oxo (oxoferriooxy) iron. phosphorus(iii) oxide is unlikely to be reacted directly with a base. iron(iii) oxide has the chemical formula {eq}fe_{2}o_{3} {/eq}. In phosphorous acid, the two hydrogen atoms. iron iii oxide is generally basic and it forms a weak base solution when. Iron Iii Oxide Acid Or Base.
From www.sciencephoto.com
Iron (III) oxide in water and in hydrochloric acid Stock Image C036/3133 Science Photo Library Iron Iii Oxide Acid Or Base phosphorus(iii) oxide is unlikely to be reacted directly with a base. iron(iii) oxide has the chemical formula {eq}fe_{2}o_{3} {/eq}. so fe +2 is iron (ii) and fe +3 is iron (iii). ferric oxide, also known as iron(iii) oxide, is an amphoteric oxide of iron. chemical properties of iron(iii) oxide reaction with acids. Iron(iii) oxide reacts. Iron Iii Oxide Acid Or Base.
From noahchemicals.com
3 Manufacturing Applications for Iron (III) Oxide Noah Chemicals Iron Iii Oxide Acid Or Base Some of the metals form very common ions which have latin names that are in common use,. iron iii oxide is generally basic and it forms a weak base solution when it gets completely dissolved in water. chemical properties of iron(iii) oxide reaction with acids. ferric oxide, also known as iron(iii) oxide, is an amphoteric oxide of. Iron Iii Oxide Acid Or Base.
From www.researchgate.net
Mineral pigments in the iron(III) oxide/hydroxide system. Download Scientific Diagram Iron Iii Oxide Acid Or Base It is also referred to as ferric oxide, red iron oxide, iron trioxide, and oxo (oxoferriooxy) iron. iron (iii) oxide is a compound that appears in at least four different polymorphs: Iron(iii) oxide reacts with acids, such as hydrochloric acid, to form iron(iii) chloride and water. Some of the metals form very common ions which have latin names that. Iron Iii Oxide Acid Or Base.
From www.showme.com
Iron III oxide Science, Chemistry, Chemical Bonds ShowMe Iron Iii Oxide Acid Or Base iron (iii) oxide is a compound that appears in at least four different polymorphs: ferric oxide, also known as iron(iii) oxide, is an amphoteric oxide of iron. iron iii oxide is generally basic and it forms a weak base solution when it gets completely dissolved in water. phosphorus(iii) oxide is unlikely to be reacted directly with. Iron Iii Oxide Acid Or Base.
From stock.adobe.com
Iron Iii Oxide Properties and Chemical Compound Structure Stock Vector Adobe Stock Iron Iii Oxide Acid Or Base so fe +2 is iron (ii) and fe +3 is iron (iii). chemical properties of iron(iii) oxide reaction with acids. iron(iii) oxide has the chemical formula {eq}fe_{2}o_{3} {/eq}. iron iii oxide is generally basic and it forms a weak base solution when it gets completely dissolved in water. iron (iii) oxide is a compound that. Iron Iii Oxide Acid Or Base.
From www.youtube.com
Is Fe2O3, Iron (III) oxide. Ionic or Covalent? YouTube Iron Iii Oxide Acid Or Base so fe +2 is iron (ii) and fe +3 is iron (iii). Some of the metals form very common ions which have latin names that are in common use,. phosphorus(iii) oxide is unlikely to be reacted directly with a base. iron(iii) oxide has the chemical formula {eq}fe_{2}o_{3} {/eq}. iron (iii) oxide is a compound that appears. Iron Iii Oxide Acid Or Base.
From testbook.com
Iron(III) Oxide Formula Know Its Preparation, Properties & Uses Iron Iii Oxide Acid Or Base It is also referred to as ferric oxide, red iron oxide, iron trioxide, and oxo (oxoferriooxy) iron. In phosphorous acid, the two hydrogen atoms. chemical properties of iron(iii) oxide reaction with acids. iron (iii) oxide is a compound that appears in at least four different polymorphs: iron iii oxide is generally basic and it forms a weak. Iron Iii Oxide Acid Or Base.
From www.numerade.com
SOLVEDA sample consisting of a mixture of iron and iron(III) oxide was dissolved completely in Iron Iii Oxide Acid Or Base iron (iii) oxide is a compound that appears in at least four different polymorphs: chemical properties of iron(iii) oxide reaction with acids. so fe +2 is iron (ii) and fe +3 is iron (iii). ferric oxide, also known as iron(iii) oxide, is an amphoteric oxide of iron. iron iii oxide is generally basic and it. Iron Iii Oxide Acid Or Base.
From lab.honeywell.com
Iron(III) oxide 12344 Honeywell Research Chemicals Iron Iii Oxide Acid Or Base so fe +2 is iron (ii) and fe +3 is iron (iii). Iron(iii) oxide reacts with acids, such as hydrochloric acid, to form iron(iii) chloride and water. phosphorus(iii) oxide is unlikely to be reacted directly with a base. iron (iii) oxide is a compound that appears in at least four different polymorphs: chemical properties of iron(iii). Iron Iii Oxide Acid Or Base.